Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 14, Article number: 24205 (2024)
Metrics details
This study aimed to evaluate the correlation between mental health status and arterial stiffness. A Symptom Checklist 90 (SCL-90) score was conducted for 10,688 employees of Kailuan Group Co., Ltd., of which 4936 participants received baPWV measurement. Of these, 4424 met the inclusion criteria. Based on the SCL-90 score, the study subjects were divided into normal mental health group (SCL-90 score < 160, 3993 cases) and abnormal mental health group (SCL-90 score ≥ 160, 431 cases). Statistical indicators include: General information, including levels of brachial-ankle pulse wave velocity (baPWV), age, gender, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), body mass index (BMI), smoking and alcohol consumption, daily activity levels, nature of work and educational qualifications. The proportion of males, baPWV value, and abnormal proportion of baPWV in normal mental health group were higher than those in abnormal mental health group (P < 0.05). The Hs-CRP in normal mental health group were lower than that in abnormal mental health group (P < 0.05). There were significant differences in activity level and educational attainment between the two groups (P < 0.05). After adjusting for confounders, the results of the multiple linear regression analysis showed that, Age, MAP, HR, FBG, TG were positively correlated with baPWV; SCL-90 score, gender, BMI, educational qualification were negatively correlated with baPWV. When the SCL-90 score of the general population increased by one point, baPWV decreased by 0.246 cm/s. Each such increase corresponded with a decrease in baPWV of 0.299 cm/s for male participants in general (β = − 0.299, P = 0.007) and 0.412 cm/s for the male participants in the older-age group (β = − 0.412, P = 0.017). Although adverse psychological factors have a certain impact on arterial stiffness, it does not constitute an independent risk factor.
Arterial stiffness is a sign of vascular aging1, and it is a risk factor for hard endpoints such as myocardial infarction (MI), cerebral stroke, and all-cause mortality2,3,4. It is also a risk factor for hypertension, cognitive impairment, and renal function decline5,6,7,8. Currently, aging and hypertension are known to be the main causes of arterial stiffness9,10. Pulse wave velocity (PWV) represents the velocity at which the pulse fluctuation caused by blood circulation during cardiac ejection travels to the peripheral blood vessels. PWV is proportional to the stiffness of the arterial wall and inversely proportional to the diameter of the vessel. PWV in large arteries can be used as a marker for the detection of atherosclerosis11. Several studies have shown that brachial-ankle pulse wave velocity (baPWV) is a good predictor of arterial stiffness12,13,14,15.
Previous studies have shown that individuals with adverse psychological factors such as typical type A personality, chronic stress, anxiety or depressive behaviors have an increased risk of MI or stroke by 1.57 times, 2.67 times, 1.74 times, and 1.45 times, respectively16,17,18,19. After conducting a meta-analysis involving data on 308,849 participants in a total of 148 studies with an average follow-up period of 7.5 years, Holt et al.20 proved that individuals with adequate social relations had a mortality risk 0.5 times lower than those with poor social relations. However, the reasons why adverse psychological behaviors lead to an individual’s increased cardiovascular risk are still unclear. Chronic psychological stress contributes to the development of hypertension and hypertension leads to arterial stiffness21. Previous study showed that even a brief period of mild to moderate stress, might exert significant adverse effects on arterial stiffness21. However, the instantaneous effects on arterial stiffness were not examined. On this basic, we assumed that adverse psychological factors increase the risk of events with adverse outcomes through the worsening of arterial stiffness. The Symptom Checklist 90 (SCL-90) score is one of the most well-known global mental health tests to accurately assess an individual’s psychological symptoms and reflect the individual’s overall mental health22. Herein, our study aimed to explore the correlation between the SCL-90 score and baPWV was examined and the correlation between mental health status and arterial stiffness analyzed.
The Kailuan study was a prospective cohort study based on a community’s population (Registration Number: ChiCTR2000029767). The study design and procedures have been previously described in the literature23. During 2006–2007, the first health check-up for existing and retired employees of Kailuan Group was conducted at 11 hospitals, including the Kailuan General Hospital and its subsidiary hospitals. Subsequently, the second, third, fourth, fifth, and sixth health check-ups were performed on the same population in 2008–2009, 2010–2011, 2012–2013, 2014–2015, and 2016–2017, respectively. The contents of all six health check-ups were similar. Beginning in 2010–2011, the baPWV of a few employees was measured during the annual health check-ups24,25.
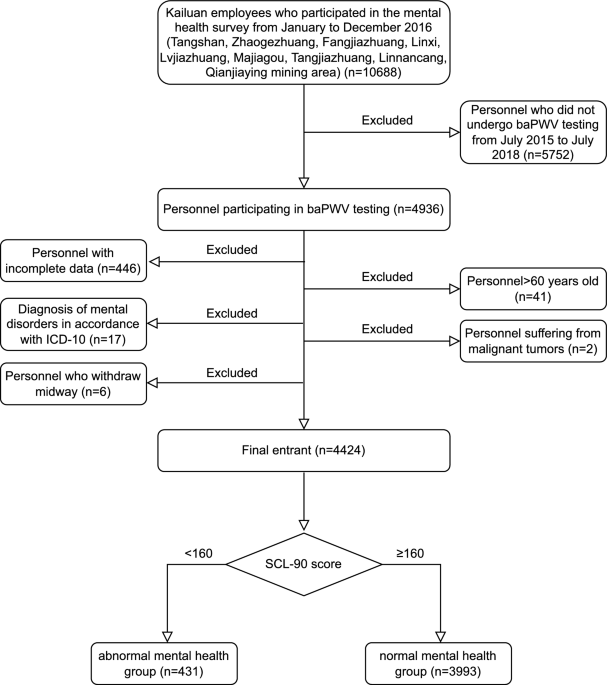
Additionally, from January to December 2016, mental health surveys were conducted for 10,688 employees of the Tangshan, Zhaogezhuang, Fangezhuang, Linxi, Lvjiatuo, Majiagou, Tangjiazhuang, Linnancang, and Qianjiaying Mines, of which 4936 participants received baPWV measurement from July 2015 to June 2018. Finally, 4424 met the inclusion criteria (Fig. 1). Based on the SCL-90 score, the study subjects were divided into normal mental health group (SCL-90 score < 160, 3993 cases) and abnormal mental health group (SCL-90 score ≥ 160, 431 cases).
Flow chart of patient enrollment.
This study was approved by the ethics committee of the Kailuan Mental Health Center (Ethical approval number: KJK-KY-2018-05-09), and the informed consent forms were obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
(i) Participants were between 18 and 60 years of age; (ii) All participants underwent physical examination and completed the SCL-90 assessment in 2016; (iii) Participants’ baPWV was measured between July 2015 and June 2018; (iv) Participants agreed to participate in the study and signed an informed consent form.
(i) Participants had a history of cancer, leukemia, and other malignancies, (ii) Participants with a variety of mental disorders that met ICD-10 diagnostic criteria (except for alcohol and nicotine dependence). (iii) Participants with hypertension, diabetes, diseases of respiratory system, cardiovascular diseases, or cerebrovascular diseases. (iv) Participants who were taking hypoglycemic drugs or hypertension medicine. (v) Participant data were incomplete.
The BP-203RPE III networked arterial stiffness detector (Omron Healthcare (China) Co., Ltd.) was used to acquire the baPWV data. The temperature of the examination room was maintained at 22–25 °C (Celsius). Prior to measurement, the participants were instructed not to smoke, to wear clothes made of a thin material, and to rest for more than 5 min. They were then asked to remain quiet, lie down supine without a pillow, and place both hands at the sides of their body with their palms facing up. The blood pressure cuffs for the four limbs were placed on the upper arms and near the ankles. For the upper arms, the airbag logo of each cuff was aligned with the brachial artery, with the lower edge of the cuff being 2–3 cm away from the cubital fossa. For the lower limbs, the airbag logo of each cuff was aligned with the medial side of the limb, with the lower edge of the cuff being 1–2 cm away from the medial malleolus. The electrocardiogram detection devices were placed on the participants’ precordial region and clipped on the left and right wrists. Two measurements were taken for each participant, and the larger baPWV value on the left or right side of the two measurements was taken as the final result. The judgment standard of the American Heart Association’s Medical Scientific Statement 1993 was used as reference to determine that baPWV ≥ 1400 cm/s was an abnormal arterial condition and indicative of arterial stiffness26.
The unit to which the research participants belonged would organize 200–300 employees to attend each assessment session, where five professional psychiatrists were in attendance to provide on-site group guidance. After the participants completed the questionnaire on their own, two psychiatrists used the Epidata3.1 software to make a double entry of the data into the system. A total score of 160 was the cut-off value based on the scoring explanation; those who scored 160 or higher and below 160 were categorized into groups with abnormal and normal mental health, respectively27.
The participants were instructed to fast for more than 8 h (h) before 5 ml (ml) of cubital venous blood was collected between 07:00 and 09:00. The serum was separated and extracted within 4 h to detect the levels of triglycerides (TG), high- and low-density lipoprotein cholesterol (HDL and LDL), fasting blood glucose (FBG), and high-sensitivity C-reactive protein (Hs-CRP). The readings were made using the automatic biochemical analyzer (Hitachi 7600, Hitachi Limited, Tokyo, Japan) and the operations were carried out by professional inspectors, strictly in accordance with the reagent instructions, with a batch-wise quality control.
Blood pressure (BP) was measured on the left arm using a mercury sphygmomanometer with an appropriately sized cuff, following the standard recommended procedures. Systolic blood pressure (SBP) is the point at which the first of two or more Korotkoff sounds is heard, and the disappearance of the Korotkoff sound indicates diastolic blood pressure (DBP). At least two readings each of SBP and DBP were taken at 5-min intervals after the participants had rested in a chair for at least 5 min. The average value of the multiple BP measures was used for further analysis. The mean arterial pressure (MAP) calculation formula is (SDP + 2 × DBP)/3.
Heart rate (HR), weight, and height were collected by licensed doctors, strictly in accordance with the measurement standards, and the body mass index (BMI) was calculated as weight (kg)/height (m)2.
The median age of 44 years was adopted as the cut-off value, following the World Health Organization’s (WHO) (1982)26 standards. Participants 44 years old and younger and those over 44 years old were categorized into the low- and high-age groups, respectively.
The smoking level was an average of at least one cigarette/day over the past year.
Alcohol consumption was an average of at least two standard glasses/day (1 g of pure alcohol ≈ 0.075 standard glass) over the past year.
The activity level was defined based on participants’ average time spent seated per day: less than 4h, between 4 and 8h, and 8h or more, which were considered as high, average, and low activity levels, respectively.
Participants were divided into physical and mental labor types based on the nature of their primary work.
Participants were divided into Junior high school and below, High school, College and above according to their educational background.
SAS v9.4 statistical software was used for the analysis. The distribution of all quantitative data was non-normal and expressed by M (Q1–Q3). The non-parametric Wilcoxon test was used to compare the inter-group differences. A multiple linear regression model and a multiple logistic regression model were then used to analyze the impact of the SCL-90 score on baPWV and the correlation between mental health status and arterial stiffness, respectively.
This study was approved by the ethics committee of the Kailuan Mental Health Center (Ethical approval number: KJK-KY-2018-05-09), and the informed consent forms were obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
There were 3321 male participants, accounting for 75.07% of the total of 4424 employees. The participants’ median age at the time of the SCL-90 assessment in 2016 was 44 (37–50). The median SCL-90 score of the total population was 106 (97–125), with the median scores of the male and female participants being 104 (96–123) and 110 (99–132), respectively. Among the 4424 participants, there were 296 (6.69%) males and 135 (3.05%) females with abnormal mental health.
The proportion of males, baPWV value, and abnormal proportion of baPWV in normal mental health group were higher than those in abnormal mental health group, the difference was statistically significant (P < 0.01). There were significant differences in activity level and educational attainment between the two groups (P < 0.01). The Hs-CRP in normal mental health group were lower than that in abnormal mental health group, the difference was statistically significant (P < 0.05). However, there were no statistically significant differences between the two groups in age, SBP, DBP, MAP, BMI, HR, FBG, HDL, LDL, TG, nature of work, and use of nicotine and alcohol (P > 0.05) (Table 1).
Considering the baPWV and the SCL-90 scores as the dependent and independent variables, respectively, stepwise (forward) multiple linear regression analysis was performed to adjust for confounders such as gender, age, MAP, BMI, HR, FBG, HDL, LDL, TG, Hs-CRP, activity level, nature of work, educational qualification, and use of nicotine and alcohol. The results indicate that, Age, MAP, HR,FBG, TG were positively correlated with baPWV (β = 8.424, 6.415, 3.323, 14.024, 4.466, respectively, P < 0.001, < 0.001, < 0.001, < 0.001, 0.011, respectively), SCL-90 score, gender, BMI, educational qualification were negatively correlated with baPWV (β = − 0.246, − 92.379, − 2.813, − 11.040, respectively, P = 0.009, < 0.001, 0.006, 0.028, respectively) (Table 2). After dividing the gender sub-groups and adjusting for confounders, when the SCL-90 score of the male participants increased by one point, baPWV decreased by 0.299 cm/s (β = − 0.299, P = 0.007) for male participants in general and 0.412 cm/s (β = − 0.412, P = 0.017) for males in the high-age group. However, there was no effect on the female and male participants in the low-age group (β = − 0.033, − 0.181, respectively, P = 0.851, 0.182, respectively) (Table 3).
For logistic regression analysis, the dependent variable was arterial stiffness (values of 1 and 0 were assigned when baPWV ≥ 1400 cm/s and < 1400 cm/s, respectively), while the independent variable was mental health status (values of 1 and 0 were assigned when SCL-90 was ≥ 160 and < 160, respectively). Three models were applied:
Model 1: A single-factor model.
Model 2: Gender and age were adjusted using Model 1 as the base.
Model 3: MAP, BMI, HR, FBG, HDL, LDL, TG, Hs-CRP, activity level, nature of work, educational qualification, and use of nicotine and alcohol were adjusted using Model 2 as the base.
Additionally, logistic regression analysis was performed on each sub-group based on gender and age.
For the general population, logistic regression analysis using single-factor Model 1 showed that poorer mental health was a risk factor for arterial stiffness (OR 1.323, 95% CI 1.081–1.619, P = 0.007). After the respective adjustments were made for Models 2 and 3, no correlation was found between mental health and arterial stiffness (OR 1.218, 95% CI 0.932–1.592, P = 0.149) (Table 4).
Similarly, no correlation was found between mental health and arterial stiffness in both male and female population after adjustment for Models 2 and 3 (OR 1.235, 1.064, respectively, 95% CI 0.922–1.654, 0.551–2.055, respectively, P = 0.156, 0.852, respectively) (Table 4).
After stratification by gender and age, we found no correlation between mental health status and arterial stiffness for each sub-group (OR 1.137, 1.296, 0.654, 1.534, respectively, 95% CI 0.746–1.737, 0.862–1.949, 0.246–1.738, 0.630–3.733, respectively, P = 0.551, 0.213, 0.395, 0.346, respectively) (Table 5).
In this study, we were unable to prove the hypothesis that adverse psychological factors lead to the worsening of arterial stiffness. Although the results of the linear regression analysis showed that the adverse psychological factors had a certain impact on the arterial stiffness, that impact did not constitute an independent risk factor. Therefore, we think that adverse psychological factors may increase the risk of adverse outcomes through other channels (e.g., lifestyle).
The results showed that the median SCL-90 score of the participants was 106 (97–125), which was lower than China’s standard of 129.96 ± 38.727. Participants with a score of 160 or higher accounted for 9.47% of the population, which was relatively low compared to the results of previous studies (3.79–29.1%)28. This situation might be because the research population was comprised entirely of employees of state-owned enterprises. These enterprises provide various guarantee mechanisms, which enable the employees to maintain living situations that were relatively stable. As a result, the employees face less social stress such as employment, income, and various types of basic insurance. Therefore, the mental health of these employees would generally be good.
The median baPWV of all participants was 1381 (1242–1555) cm/s, and those who having baPWV ≥ 1400 cm/s accounting for 46.81%. This value was lower than the 43.74–63.50%, detected during previous studies29,30,31,32 possibly due to the average age of the participants, which was relatively low, with a median age of 44 (37–50). In addition, the overall level of arterial stiffness was not high may be due to the majority of the population was engaged in heavy manual labor.
The results of previous research suggested that individuals with poor mental health were more likely to suffer from cardiovascular diseases33,34. A number of previous studies35,36 have showed that the worse the mental health and the longer the duration of the condition, the more serious the cardiovascular diseases and the higher the risk of an adverse outcomes. However, many aspects of the correlation between adverse psychological factors and the occurrence of arterial stiffness and cardiovascular diseases remain unclear. For example, Nicholson et al.37 conducted a meta-analysis of 54 observational studies that included a total of 146,533 samples and 6362 outcomes, the results showed that symptoms of depression had a clear impact on cardiovascular diseases. However, because screening for risk factors for these disorders involves subjective selection, they argued that the overall adjustment effect for depression would be weak if all etiologic factors were adjusted. Thus, depression still cannot be considered an independent risk factor for cardiovascular diseases. One the other hand, although the studies by Steptoe et al. similarly demonstrated that depression was associated with an increased risk of cardiovascular disease38, and further analyzed the relationship between work stress and arterial stiffness39, studies of this nature typically include a broader demographic and do not focus on a specific group as this study does, such as coal miners in China. Additionally, they do not employ a specific psychological assessment tool like the SCL-90 utilized in our research.
In this study, regardless of the influence of confounders, we noticed that baPWV exhibited a gradual downward trend with increasing SCL-90 scores. This was applicable to the general population but was more prominent among the male population, especially those over 44 years old. However, the results of the logistic regression analysis showed that it not an independent risk factor of arterial stiffness. It was similar to the Nicholson et al.’ study37.
The participants in this study were 18–60 years old, with a median age of 44 (37–50) years. The majority of the participants were coal miners who performed heavy manual labor and would be uniquely affected by the underground working environment. Each day, these workers were exposed to sunlight for only a short period of time, while remaining in a dim, noisy, dusty, and vibrating environment with high temperatures and humidity for a long period of time. These conditions could easily lead to a state of depression. Most coal miners have formed unique emotional traits and adopted a special lifestyle to relieve stress and relaxation, such as resting, inhaling nicotine or drinking alcohol. The proportion of smokers in this study was 45.41%, that of male smokers being 58.21%. This is double the proportion of smokers in the` European region (29%) (WHO, 2019)40. The proportion of alcohol drinkers was 58.68%, with that of male drinkers being 71.15%. This is also much higher than the 43% of alcohol drinkers worldwide (WHO, 2018)41.
The dietary structure of colliery workers mainly consists of foods high in fat, sugar, and calories to provide the additional energy for engaging in physically demanding tasks, and mealtimes are often irregular. But their eating habits would eventually become a double-edged sword. On one hand, they were able to avoid experiencing poor mental states and enjoy greater psychological pleasure, leading to a greater sense of happiness and satisfaction42,43,44; on the other hand, a high burden was placed on their cardiovascular system, which would eventually cause the worsening of arterial stiffness45,46. Of course, this hypothesis requires further analysis based on more detailed information about their dietary structure. In addition, some people were affected by their own cultural literacy and social class, had strong self-discipline, and would not eat high-sugar and high-oil diets to relieve pressure, so their dietary structure had less pressure on the cardiovascular system. This was mainly reflected in the population with college degree and above, although the proportion of these people with negative emotions was higher (44.65%), the overall level of arterial stiffness was relatively low.
The correlation between adverse psychological factors and arterial stiffness was mainly reflected for male participants over 44 years old. A possible reason is that the various compensation mechanisms of the human body are more effective at 44 years old or younger, such that the organism can better offset the impacts of the aforementioned coping style. This style do not essentially change with age, but the functionality of the various organs gradually declined and the compensation mechanisms slowly weakened. As one grows older, the body will progressively lose the ability to offset the effects exerted by various factors on the cardiovascular system, leading to more obvious abnormalities in arterial stiffness.
Overall, the population of this study had dissimilar characteristics from other populations in terms of their mental needs and lifestyle. In addition to improving the working environment to ameliorate workers’ adverse emotional states, more attention should be paid to the way they cope with negative emotions. As such, they should be guided to find alternative ways to relieve stress instead of having an unhealthy lifestyle and poor dietary structure. Meanwhile, they should be provided with more venues, conditions, and opportunities that would eventually allow them to form a different coping style with good adaptability. The resultant behaviors will ensure they will be able to maintain good mental health without any adverse effects on the cardiovascular and other systems, which is important to the overall health of this group.
There were certain limitations to this study. First, the causal relationship between mental health and arterial stiffness could not be determined by the cross-sectional study design. Second, a more detailed stratification between mental health and arterial stiffness could not be identified because there was no exhaustive survey on participants’ lifestyle and perceived stress. These limitations will be addressed in a follow-up study.
The results of this study suggested that although adverse psychological factors have a certain impact on arterial stiffness, it does not constitute an independent risk factor. Adverse psychological factors increase the risk of adverse outcomes may through other ways. In addition, more places, conditions and opportunities should be provided for people so that they can maintain good mental health to reduce the adverse effects of bad emotions on cardiovascular and other systems.
The data used to support the findings of this study are available from the corresponding author upon request.
Cavalcante, J. L., Lima, J. A., Redheuil, A. & Al-Mallah, M. H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 57, 1511–1522. https://doi.org/10.1016/j.jacc.2010.12.017 (2011).
Article PubMed Google Scholar
Mitchell, G. F. et al. Arterial stiffness and cardiovascular events: The Framingham Heart study. Circulation 121, 505–511. https://doi.org/10.1161/circulationaha.109.886655 (2010).
Article PubMed PubMed Central Google Scholar
Mattace-Raso, F. U. et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam study. Circulation 113, 657–663. https://doi.org/10.1161/circulationaha.105.555235 (2006).
Article PubMed Google Scholar
Vlachopoulos, C., Aznaouridis, K. & Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 55, 1318–1327. https://doi.org/10.1016/j.jacc.2009.10.061 (2010).
Article PubMed Google Scholar
Mitchell, G. F. et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart study. Hypertension 43, 1239–1245. https://doi.org/10.1161/01.HYP.0000128420.01881.aa (2004).
Article CAS PubMed Google Scholar
Mitchell, G. F. et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The age, gene/environment susceptibility—Reykjavik study. Brain 134, 3398–3407. https://doi.org/10.1093/brain/awr253 (2011).
Article PubMed PubMed Central Google Scholar
Taniguchi, Y. et al. Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. J. Epidemiol. 25, 592–599. https://doi.org/10.2188/jea.JE20140250 (2015).
Article PubMed PubMed Central Google Scholar
Sedaghat, S. et al. Arterial stiffness and decline in kidney function. Clin. J. Am. Soc. Nephrol. 10, 2190–2197. https://doi.org/10.2215/cjn.03000315 (2015).
Article PubMed PubMed Central Google Scholar
Lin, L. Y. et al. Determinants of arterial stiffness progression in a Han-Chinese population in Taiwan: A 4-year longitudinal follow-up. BMC Cardiovasc. Disord. 15, 100. https://doi.org/10.1186/s12872-015-0093-2 (2015).
Article PubMed PubMed Central Google Scholar
Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 65, 252–256. https://doi.org/10.1161/hypertensionaha.114.03617 (2015).
Article CAS PubMed Google Scholar
Shen, L. et al. Relationship between pulse wave velocity and carotid atherosclerosis in geriatric people. Cerebrovasc. Dis. 32(Suppl 1), 16–20. https://doi.org/10.1159/000330316 (2011).
Article PubMed Google Scholar
Majdouline, Y. et al. Endovascular shear strain elastography for the detection and characterization of the severity of atherosclerotic plaques: In vitro validation and in vivo evaluation. Ultrasound Med. Biol. 40, 890–903. https://doi.org/10.1016/j.ultrasmedbio.2013.12.008 (2014).
Article PubMed Google Scholar
Ninomiya, T. et al. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: The Hisayama study. J. Hypertens. 31, 477–483. https://doi.org/10.1097/HJH.0b013e32835c5c23 (2013).
Article CAS PubMed Google Scholar
Lee, J. Y. et al. Association between brachial-ankle pulse wave velocity and progression of coronary artery calcium: A prospective cohort study. Cardiovasc. Diabetol. 14, 147. https://doi.org/10.1186/s12933-015-0311-3 (2015).
Article CAS PubMed PubMed Central Google Scholar
Ishisone, T. et al. Comparison of utility of arterial stiffness parameters for predicting cardiovascular events in the general population. Int. Heart J. 54, 160–165. https://doi.org/10.1536/ihj.54.160 (2013).
Article PubMed Google Scholar
O’Connor, N. J., Manson, J. E., O’Connor, G. T. & Buring, J. E. Psychosocial risk factors and nonfatal myocardial infarction. Circulation 92, 1458–1464. https://doi.org/10.1161/01.cir.92.6.1458 (1995).
Article CAS PubMed Google Scholar
Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364, 937–952. https://doi.org/10.1016/s0140-6736(04)17018-9 (2004).
Article PubMed Google Scholar
Martens, E. J. et al. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease: The Heart and Soul study. Arch. Gen. Psychiatry 67, 750–758. https://doi.org/10.1001/archgenpsychiatry.2010.74 (2010).
Article PubMed Google Scholar
Pan, A., Sun, Q., Okereke, O. I., Rexrode, K. M. & Hu, F. B. Depression and risk of stroke morbidity and mortality: A meta-analysis and systematic review. JAMA 306, 1241–1249. https://doi.org/10.1001/jama.2011.1282 (2011).
Article CAS PubMed PubMed Central Google Scholar
Holt-Lunstad, J., Smith, T. B. & Layton, J. B. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 7, e1000316. https://doi.org/10.1371/journal.pmed.1000316 (2010).
Article PubMed PubMed Central Google Scholar
Logan, J. G. et al. Acute psychological stress, autonomic function, and arterial stiffness among women. Int. J. Psychophysiol. 155, 219–226 (2020).
Article PubMed PubMed Central Google Scholar
Derogatis, L. R. The Brief Symptom Inventory (BSI) Administration, Scoring, and Procedures Manual (National Computer Systems, 1993).
Google Scholar
Wu, S. et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ. Cardiovasc. Qual. Outcomes 5, 487–493. https://doi.org/10.1161/circoutcomes.111.963694 (2012).
Article PubMed Google Scholar
Ma, C. et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: A prospective study. Neurology 93, e445–e457. https://doi.org/10.1212/wnl.0000000000007853 (2019).
Article CAS PubMed PubMed Central Google Scholar
Wu, S. et al. Aging, arterial stiffness, and blood pressure association in Chinese adults. Hypertension 73, 893–899. https://doi.org/10.1161/hypertensionaha.118.12396 (2019).
Article CAS PubMed Google Scholar
Yamashina, A. et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens. Res. 26(8), 615–622 (2003).
Article PubMed Google Scholar
Zhang, Z. J. Manual of behavioral medicine scales (special issue). Chin. J. Behav. Med. Sci. 14(6), 517 (2005).
Google Scholar
Tang, Q. P., Cheng, Z. H., Yuan, A. H. & Deng, Y. L. The use and reanalysis of SCL-90 in China. Chin. J. Clin. Psychol. 01, 19–23 (1999).
Google Scholar
Fan, G. Y. et al. Analysis of brachial-ankle pulse wave velocity for 20748 subjects undergoing physical examination. Chin. J. Arterioscler. 22(8), 803–807 (2022).
Google Scholar
Wang, L. M. et al. The association between BMI trajectories and brachial-ankle pulse wave velocity. Chin. J. Arterioscler. 25(10), 1041–1046. https://doi.org/10.3969/j.issn.1007-3949.2017.10.014 (2017).
Article Google Scholar
Zhao, M. & Cai, M. X. Association of brachial-ankle pulse wave velocity and other risk factors of cardiovascular disease in health check examinees. Mod. Prev. Med. 45(10), 1904–1907 (2018).
Google Scholar
Liu, Z., Zhang, Y., Zhao, Y. L. & Wang, H. The regression analysis of brachial-ankle pulse wave velocity and cardiovascular risk factors. J. Cardiovasc. Pulm. Dis. 36(2), 90–92. https://doi.org/10.3969/j.issn.1007-5062.2017.02.004 (2017).
Article Google Scholar
Mannie, Z. N. et al. Cardiovascular and metabolic risk profile in young people at familial risk of depression. Br. J. Psychiatry 203, 18–23. https://doi.org/10.1192/bjp.bp.113.126987 (2013).
Article PubMed Google Scholar
Scott, K. M. et al. Associations between DSM-IV mental disorders and subsequent heart disease onset: Beyond depression. Int. J. Cardiol. 168, 5293–5299. https://doi.org/10.1016/j.ijcard.2013.08.012 (2013).
Article PubMed Google Scholar
Seldenrijk, A. et al. Depression, anxiety, and arterial stiffness. Biol. Psychiatry 69, 795–803. https://doi.org/10.1016/j.biopsych.2010.12.034 (2011).
Article PubMed Google Scholar
Doyle, F. et al. Systematic review and individual patient data meta-analysis of sex differences in depression and prognosis in persons with myocardial infarction: A MINDMAPS study. Psychosom. Med. 77, 419–428. https://doi.org/10.1097/psy.0000000000000174 (2015).
Article PubMed Google Scholar
Nicholson, A., Kuper, H. & Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146,538 participants in 54 observational studies. Eur. Heart J. 27, 2763–2774. https://doi.org/10.1093/eurheartj/ehl338 (2006).
Article PubMed Google Scholar
Kivimäki, M. & Steptoe, A. Depression and cardiovascular disease: A systematic review and meta-analysis. Am. Coll. Cardiol. 71(23), 2629–2642 (2018).
Google Scholar
Steptoe, A. et al. Association between job strain and arterial stiffness: A systematic review and meta-analysis. Am. Heart Assoc. 8(2), e010826 (2019).
Google Scholar
World Health Organization. European Tobacco Use. https://www.euro.who.int/__data/assets/pdf_file/0009/402777/Tobacco-Trends-Report-ENG-WEB.pdf (World Health Organization).
World Health Organization. Global Status Report on Alcohol and Health. https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf?ua=1 (World Health Organization, 2018).
Macht, M. How emotions affect eating: A five-way model. Appetite 50(1), 1–11 (2008).
Article PubMed Google Scholar
Rosenstein, D. & Oster, H. Differential facial responses to four basic tastes in newborns. Child Dev. 59, 1555–1568 (1988).
Article CAS PubMed Google Scholar
Steiner, J. E. Human facial expressions in response to taste and smell stimulation. Adv. Child Dev. Behav. 13, 257–295 (1979).
Article CAS PubMed Google Scholar
Tsai, J. P. & Hsu, B. G. Arterial stiffness: A brief review. Tzu Chi Med. J. 33(2), 115–121 (2020).
Article PubMed PubMed Central Google Scholar
Shirwany, N. A. & Zou, M. H. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 31(10), 1267–1276 (2010).
Article CAS PubMed PubMed Central Google Scholar
Download references
The authors would like to thank Professor Shouling Wu for his guidance during the course of this project. The authors also thank all the members of the Kailuan Mental Health Center for their contribution and the participants who contributed their data.
Administrative Office, Kailuan Mental Health Centre, Tangshan, Hebei, China
Shun Zhang, Liping Wang, Wenyou Ma, XiaoLiang Liang, Yan Sun & Zhenjian Yu
The Fourth Ward, Kailuan Mental Health Centre, Tangshan, Hebei, China
Na Li
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
Conceptualization: Shun Zhang, Wenyou Ma Data Curation and Investigation: Na Li, Xiaoliang Liang, Yan Sun Data Analysis: Shun Zhang, Zhenjian Yu Formal Analysis: Liping Wang, Zhenjian Yu Methodology: Liping Wang, Wenyou Ma Writing—Original Draft Preparation: Shun Zhang All authors have read and approved the final version of the manuscript. Corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Correspondence to Na Li or Zhenjian Yu.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
Zhang, S., Li, N., Wang, L. et al. The correlation between mental health and arterial stiffness in Chinese population. Sci Rep 14, 24205 (2024). https://doi.org/10.1038/s41598-024-67779-z
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67779-z
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
© 2024 Springer Nature Limited
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.